
The industry and key opinion leaders recognize the SYMATESE HA KNOW-HOW

ESTYME® FILLERS for facial rejuvenation

Focus on ESTYME® LIFT

Optimized Syringe-Gel combination
Better comfort for the patient

Efficacy and safety proven with clinical trials
SYMATESE HA KNOW-HOW recognised by leaders in various fields
15 years scientific research in HA applied in facial rejuvenation and therapeutic areas
Lipoatrophy HIV Radiotherapy Ophthalmology Rheumatology
New augmented HA technology
Our milestones in medical aesthetics
- 2011 : 1st generation HA dermal Fillers launched by a world leader
- 2021 : 2nd generation : SYMATESE gains the CE marking for ESTYME LIFT® among its future 4 products range

HA ESTYME® FILLERS range
SYMATESE designed an exclusive and finely tuned dermal filler portfolio* including lidocaine with properties adapted by indication.
*ESTYME® LIFT is CE Marked.
Certification in process for the rest of the ESTYME® Fillers range, expected in 2023
ESTYME® LIFT presentation
ESTYME® LIFT is cross-linked hyaluronic acid gel from non animal sources, containing lidocaine.
ESTYME® LIFT is indicated for the correction of nasolabial folds and intended to be injected into dermis to hypodermis.
It is the first CE marked product among the ESTYME® FILLERS portfolio.
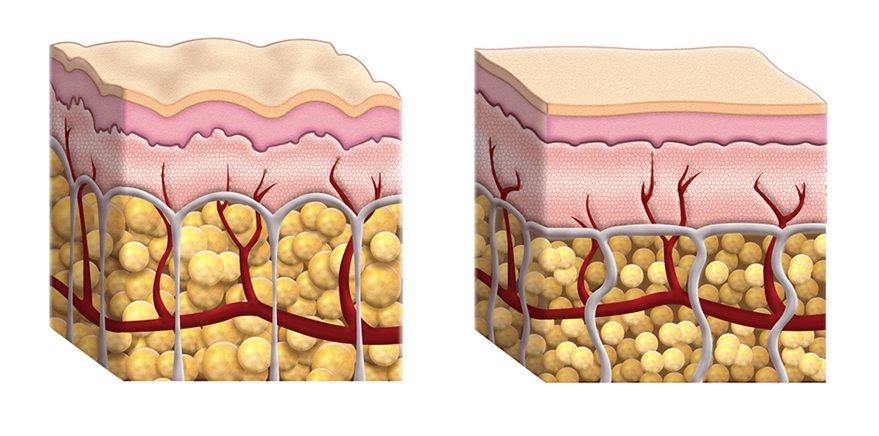
ESTYME® LIFT mode of action
ESTYME® LIFT acts by adding volume to the tissue, thereby restoring the skin contours and the volume and shape of the face to the desired level. The volume and lifting capacity originate from the ability of the cross-linked hyaluronic acid gel to hold its shape in the tissue, thus maintaining over time the volume and projection obtained at injection.
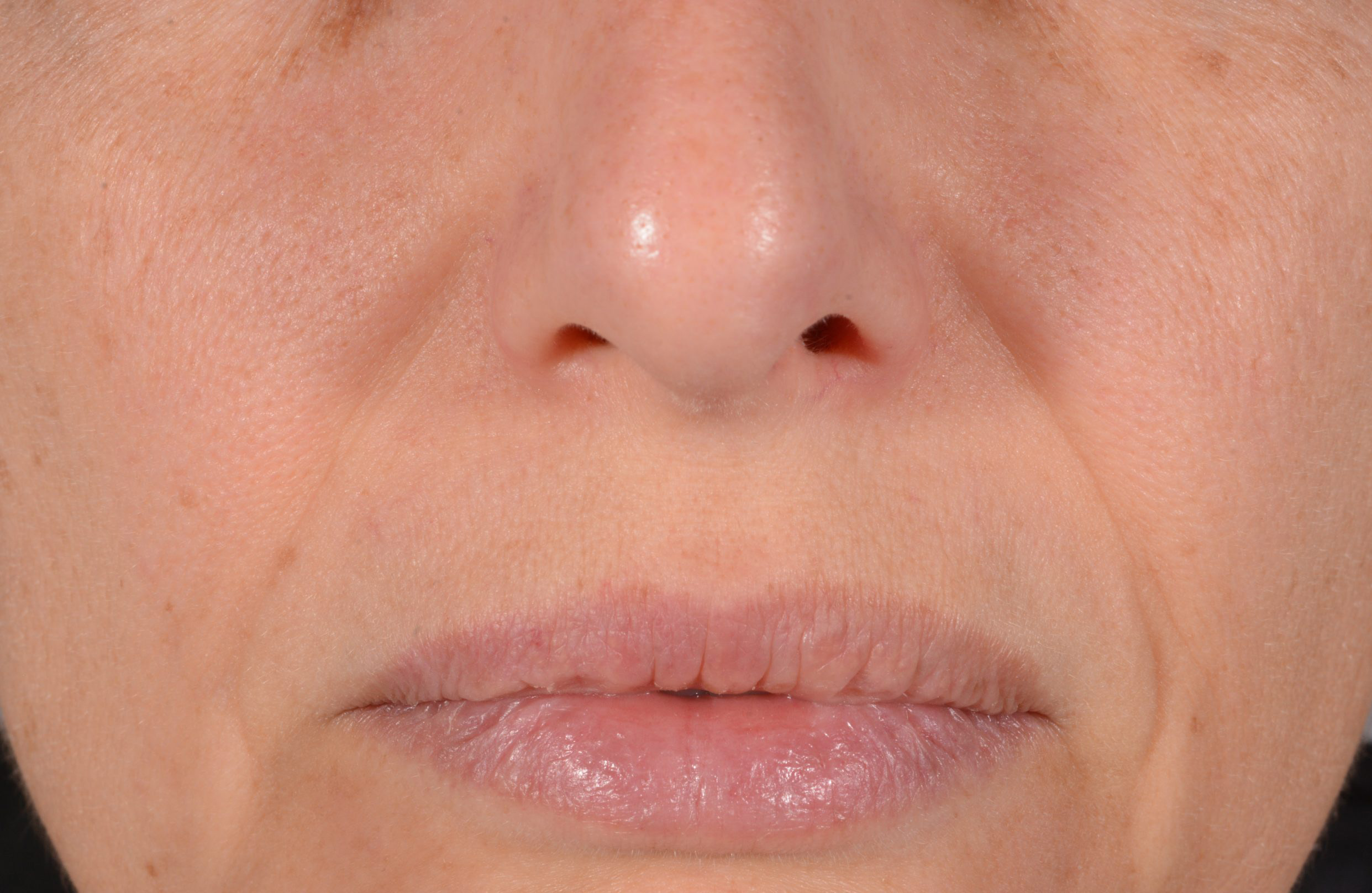
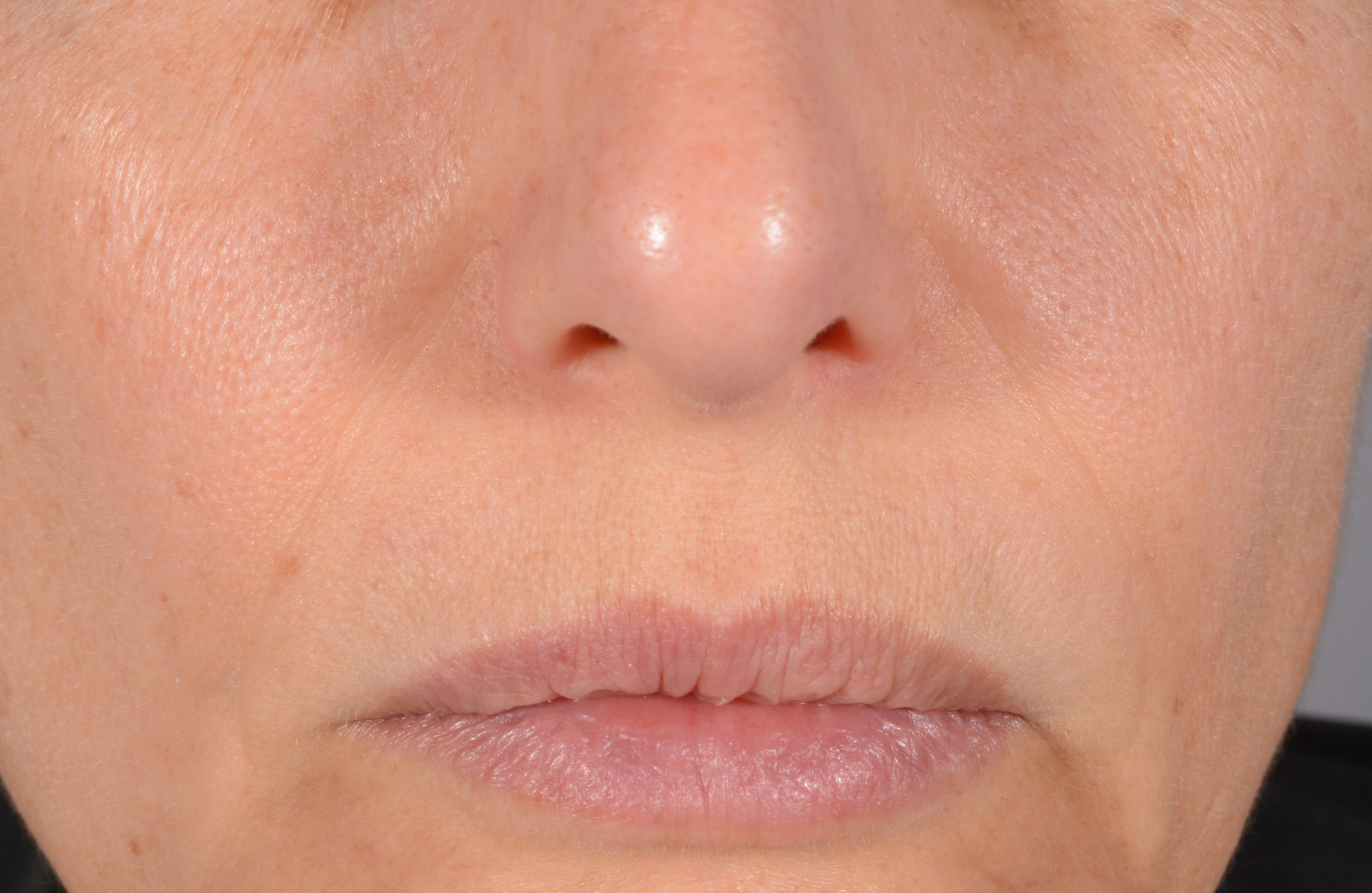
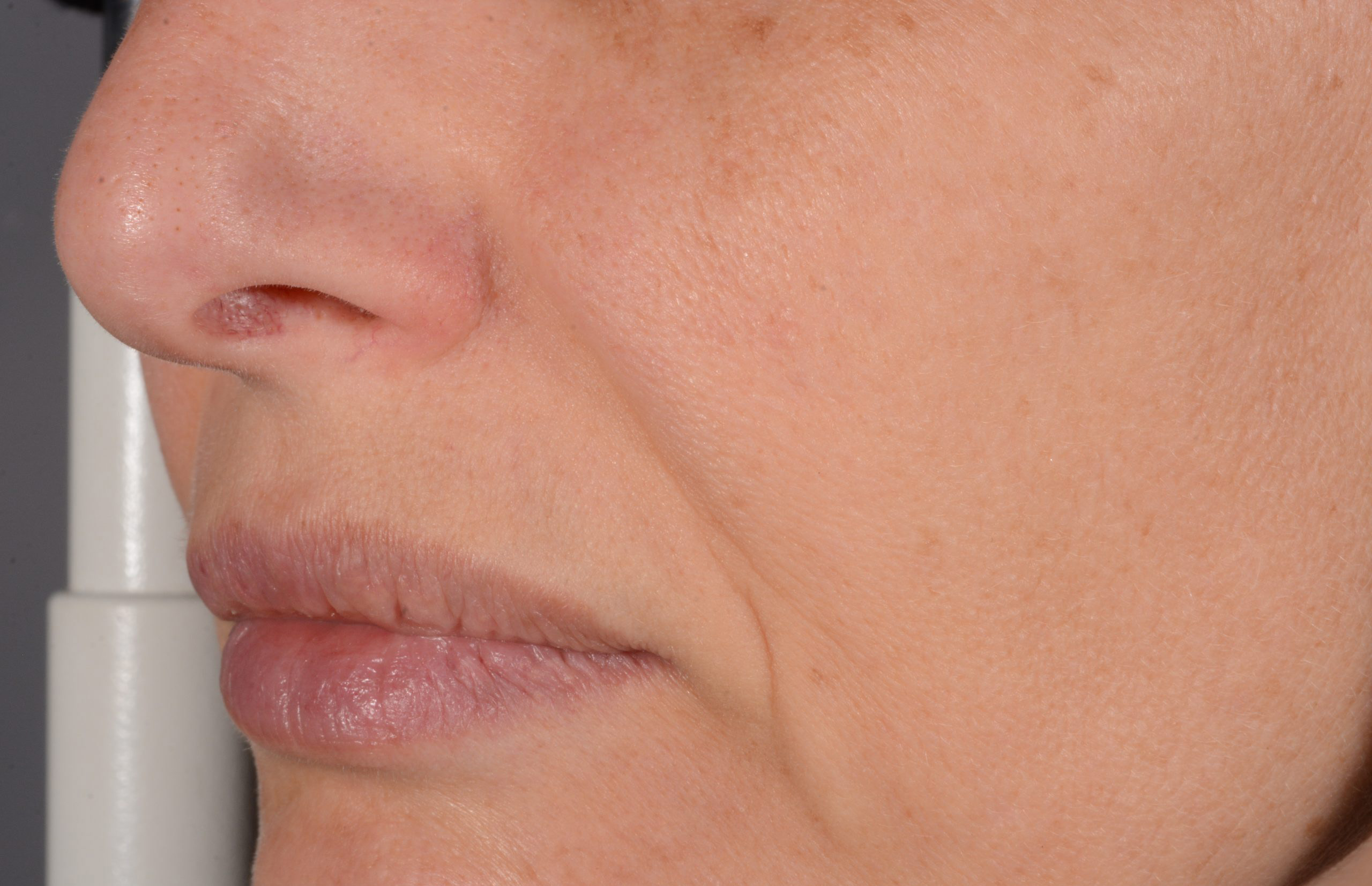
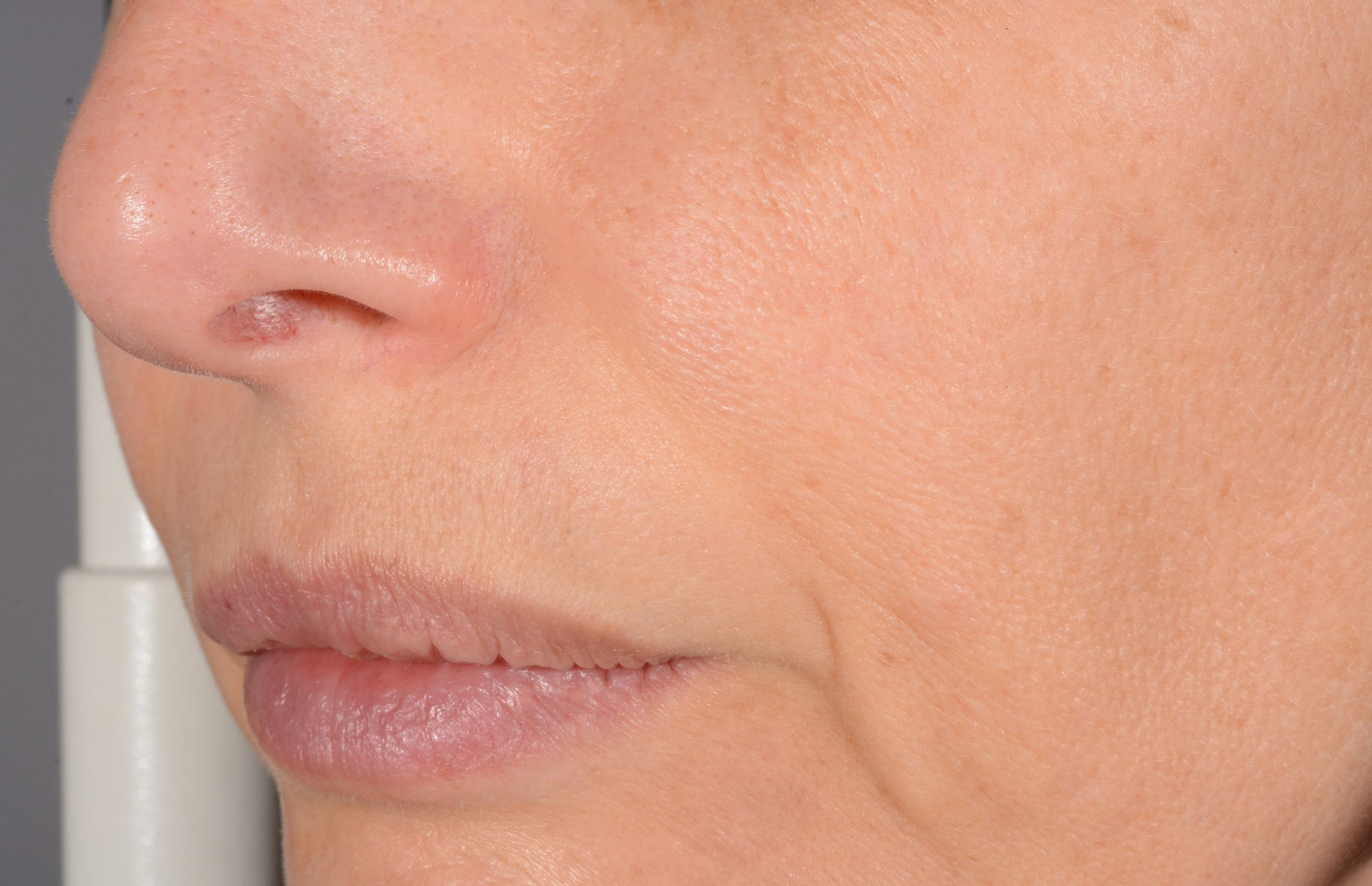
Before and after
with ESTYME® LIFT on NASIOLABIAL FOLDS
Our organisation developed and manufactured both syringes and HA gel for an ergonomic experience
The new ergonomic delivery system allows an easy flowing, for physicians through the syringe and product.
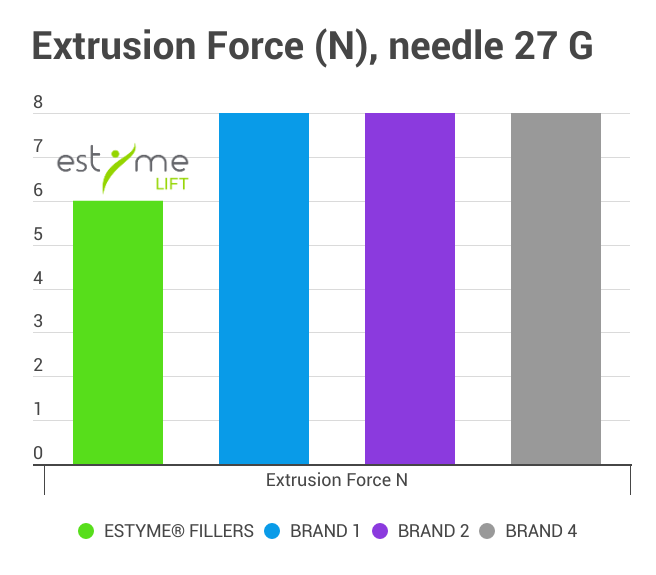
Thanks to the combination gel-syringe by the sister companies which allows a minimal extrusion force to make the injection comfortable for the physicians and its patients
A lower extrusion force
4 prospective clinical trials on 207 patients for each product in facial rejuvenation and HIV lipoatrophy
To demonstrate the exclusive safety and efficacy profile of ESTYME® FILLERS in aesthetic and medical indications.
Including one comparative randomized study on ESTYME® LIFT
- 45 patients with symmetric nasolabial folds
- from moderate (grade 3) to severe (grade 4), according to the WSRS scale.
- The mean age of subjects was 58 years
- 84% of subjects were women.
The study compares ESTYME® LIFT to a worldwide commonly used HA-lidocaine filler, approved in treatment of nasolabial folds (control). For each patient, both products are injected either on the left or on the right side.
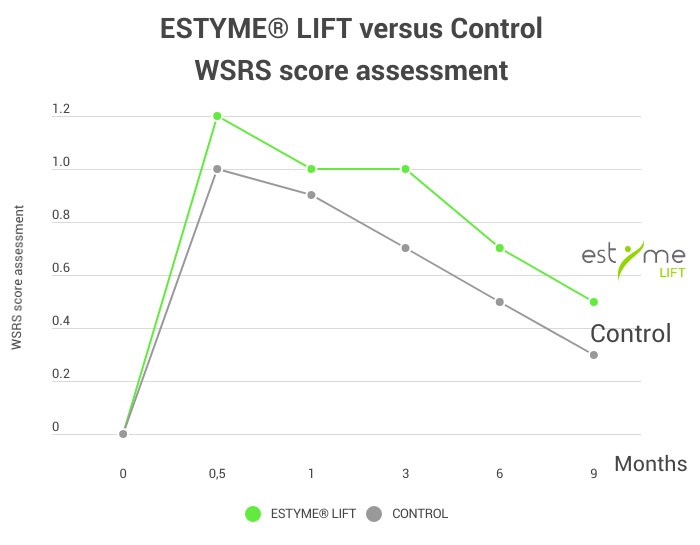
The WSRS assessment highlights an improvement in age-related folds throughout the study with a significant difference at 3 and 6 months in favor of ESTYME® LIFT as well as prolonged effectiveness by 3 months compared to the control.
Regarding safety, no significant difference was observed between both devices



